Repurposed Treatment for Cocaine Use Disorder
Application
Repurposed drug for transcriptomic-driven treatment of cocaine use disorder.
Key Benefits
- FDA-approved drug.
- Outperformed current targets undergoing clinical trials for CUD at the mRNA level.
Market Summary
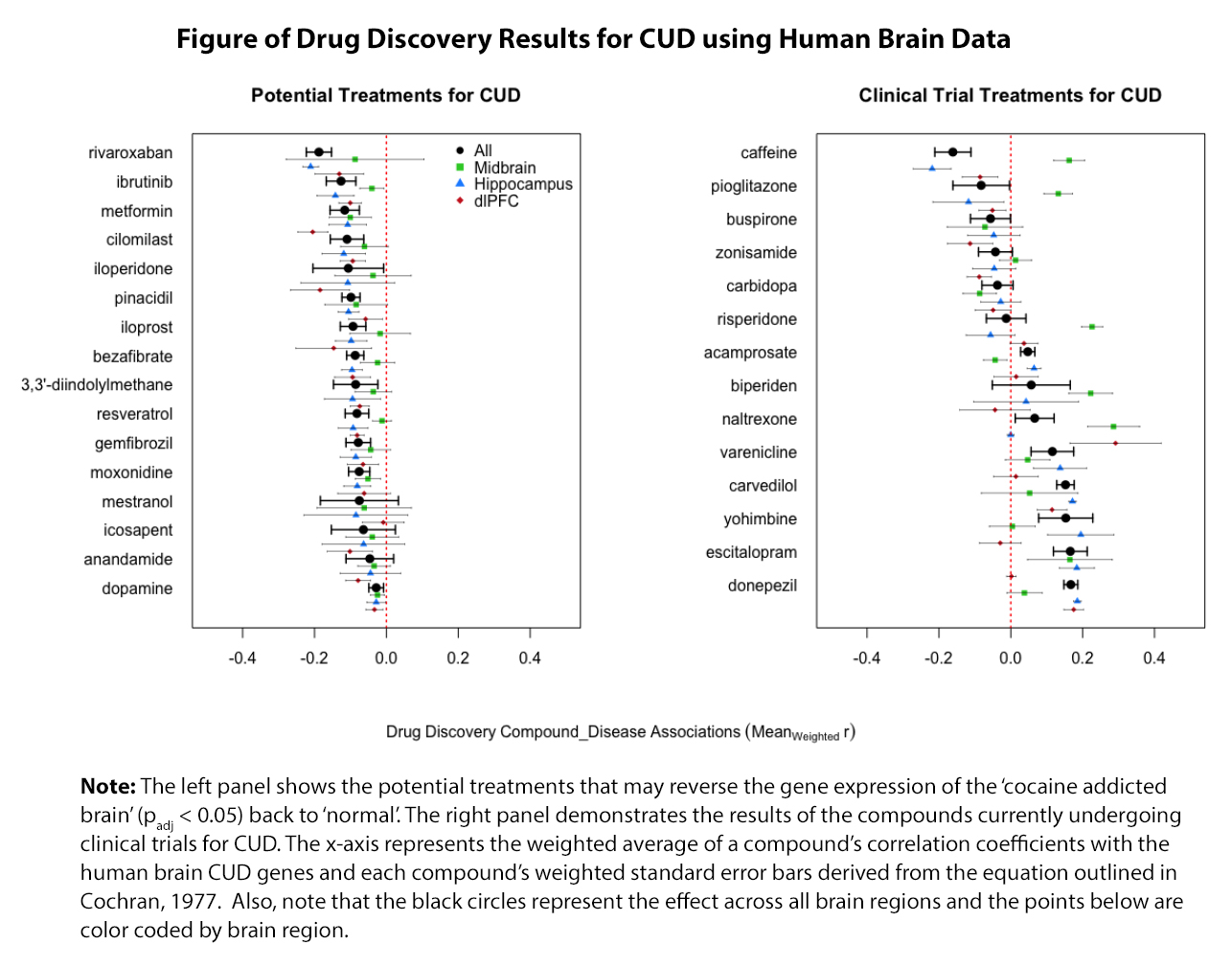
Cocaine abuse is associated with substantial morbidity and mortality, and there are no approved medications for the treatment of CUD. The current standard of treatment includes psychosocial treatments. Despite progress in the development of psychosocial approaches for CUD, many patients still do not respond to these treatments.
Technical Summary
Emory University inventors hypothesized that if the transcriptional signatures of a compound are negatively associated with a diseased state, this compound may reverse the underlying disease mechanisms back to normal. The inventors have identified repurposed medications that opposed the neuropathology of human CUD. In in vitro studies, these compounds reversed the CUD genes in human neuronal cells exposed to cocaine, as well as on cocaine self-administrated mouse. Based on validation using datasets, these compounds outperformed current targets undergoing clinical trials for CUD at the mRNA level.
Developmental Stage
- Lead compound has been identified and in vitro studies complete.
- Lead compound has undergone in vivo fruit fly testing.
- Inventors are seeking collaborators and funding.
Read our featured innovation.
Patent Information
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Patent Status |
| PCT |
PCT |
PCT/US2021/048188 |
|
8/30/2021 |
|
National Phase Entered |
| Nationalized PCT - United States |
United States |
18/023,797 |
|
2/28/2023 |
|
Pending |
| Nationalized PCT - Foreign |
EP |
21862907.9 |
|
3/20/2023 |
|
Pending |
|
|

