Application
Treatment of solid tumors combining purine nucleoside phosphorylase (PNP) technology with checkpoint blockade inhibition.
Key Benefits
- Safe and effective method for markedly enhancing checkpoint blockade therapy for refractory tumors.
- Checkpoint inhibitor therapy is FDA-approved and already in use.
Market Summary
Checkpoint blockade is an innovative form of cancer therapy that confers regressions and cures of numerous human tumor types. For certain forms of cancer, however, the benefit of checkpoint blockade is marginal. (Head and neck malignancies, triple-negative breast cancer (TNBC), others.)
Technical Summary
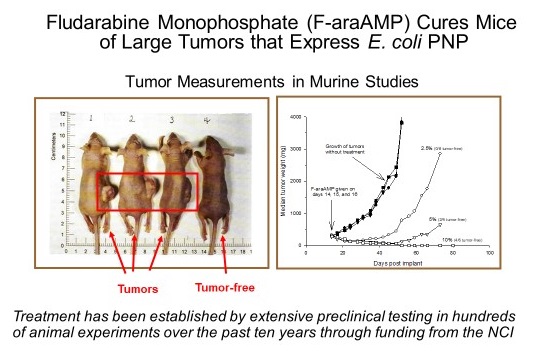
Emory University researchers have discovered that expression of the E. coli purine nucleoside phosphorylase (E. coli PNP) gene in a small percentage of cells within a solid tumor can be exploited to selectively kill the entire malignant mass. This is achieved by coupling the locally-produced PNP enzyme with a prodrug that is metabolized into an active chemotherapeutic agent with high potency. The inventors’ current PNP configuration combines E. coli PNP together with the prodrug fludarabine phosphate (F-araAMP), a chemotherapeutic agent already in clinical use. The administration of F-araAMP leads to PNP-dependent generation of a robust anti-cancer compound that ablates tumor tissue. Studies have shown that PNP-based treatment is additive or synergistic with checkpoint blockade in tumors that are otherwise very resistant. Importantly, PNP therapy also sensitizes non-PNP-treated tumors to checkpoint blockade (so-called “abscopal” effect). The new approach generates chemotherapy specifically within malignant tissue (a means to optimize safety), with any anti-cancer agent that escapes the tumor mass rapidly metabolized by a ubiquitous human enzyme, xanthene oxidase. This new combinatorial treatment produces better outcomes than monotherapy in otherwise refractory tumors.
Developmental Stage
- Phase 1 clinical trial of PNP/fludarabine, showing favorable efficacy with no significant toxicity, has been completed and published.
- Phase 2 clinical testing has been initiated in collaboration with the Stanford University Cancer Center.
- Evidence of synergy or additivity with checkpoint blockade inhibitors in murine models of triple-negative breast cancer.
- Currently seeking licensee or partnership for clinical development.
Publications
Behbahani, T. E. et al. (2018). Head & Neck, 41(6), 1979-1983.
Parker, W. B., Sorscher, E. J. (2017). Current Pharmaceutical Design, 23(45), 7003-7024.
Rosenthal, E. L. et al. (2015). Annals of Oncology, 26(7), 1481-1487.

