Targeted Theranostic NanoToxin for Treating Metastatic and Drug-resistant Tumors
Application
Magnetic theranostic nano-toxin for imaging and drug delivery applications in cancer therapy.
Key Benefits
- Induces tumor cell death via a different biological mechanism, compared to current chemotherapeutics, to overcome drug resistance mechanisms in tumor cells.
- Delivers high levels of therapeutic agents into metastatic tumors, while reducing systemic toxicity.
- Enables molecular optical and MR imaging to monitor drug delivery, therapeutic responses and intraoperative optical imaging to remove drug resistant tumor lesions.
Market Summary
Cancer patients with metastatic diseases usually have received extensive standard treatments of combinations of chemotherapy, radiotherapy, molecular targeted therapy and immunotherapy. However, the majority of the patients are resistant to the therapies or develop acquired resistance to the therapies. The prognoses of those patients are very poor. At present, cancer patients with metastatic peritoneal tumors (PC) have a poor response to systemic chemotherapy. Intraperitoneal (IP) chemotherapy in combination with surgery has been used for the treatment of some PC patients. However, this approach has limitations due to systemic toxicity, high IP drug doses, and rapid absorption of drugs into the systemic circulation. Additionally, conventional IP therapy has a low efficiency of drug penetration into bulky tumors (>1 cm). The development of novel therapeutics to enhance the targeted delivery of potent therapeutic agents into IP tumors is needed for the survival of PC patients who are currently considered untreatable.
Technical Summary
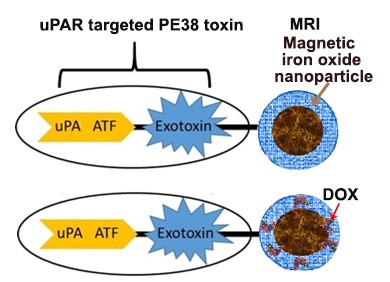
Emory inventors have developed theranostic magnetic iron oxide nanoparticles (IONPs) conjugated with a recombinant toxin protein for treating metastatic and drug-resistant tumors. The protein contains the amino acids of the receptor binding region (ATF) of the urokinase plasminogen activator (uPA) and a bacterial toxin (ATF-Toxin-IONP) to target this nanotoxin to tumor cells for effectively killing drug resistant tumor cells. The target receptor (urokinase plasminogen activator receptor) is present on tumor cells and brings the nanotoxin into tumor cells, resulting in the release of a potent modified pseudomonas exotoxin A (PE38) to block protein synthesis and induce tumor cell death. The cell death mechanism could bypass many drug resistant mechanisms in tumor cells and thereby, overcome resistance to therapy. Nanoparticle mediated targeted delivery of PE38 toxin could reduce systemic toxicity. Additional therapeutic agents, such as chemotherapy drug doxorubicin (DOX), can be encapsulated into the nanotoxin (ATF-toxin-IONP/DOX) to further enhance the therapeutic effect. These targeted nanotoxin agents allow for the selective delivery of PE38 and DOX to metastatic tumors either by systemic delivery or peritoneal administrations, to induce tumor cell death and tumor growth inhibition. In addition, the specific ATF-Toxin-IONP-Dox might be a very potent cancer therapeutic agent with a strong growth inhibition effect on both primary and metastatic tumors, but low systemic toxicity. Importantly, this nanoparticle also enables MRI monitoring of drug delivery and tumor response. Addition of an optical dye on the nanotoxin also provides an optical imaging capability for optical image-guided surgery for removal of therapy resistant tumors.
Developmental Stage
Preliminary data demonstrated targeted delivery and in vivo efficacy following systemic or intraperitoneal delivery of ATF-toxin-IONP or ATF-Toxin-IONP/DOX in human breast, colon and pancreatic cancer patient derived xenograft models.
Patent Information
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Patent Status |
| Nationalized PCT - United States |
United States |
16/651,699 |
11,633,363 |
3/27/2020 |
4/25/2023 |
Issued |
| Divisional |
United States |
18/182,682 |
12,458,603 |
3/13/2023 |
11/4/2025 |
Issued |
|
|

