Three-piece Partially Biodegradable Screw for Use in Ankle Surgery
Application
Three-piece, partially biodegradable cannulated screw for use in syndesmosis ankle surgery.
Key Benefits
- Implant removal is not necessary.
- Allows for both rigid initial fixation to allow syndesmotic healing and physiologic micro motion at the syndesmosis for long term support and function.
- Higher biomechanical strength than absorbable screws.
Market Summary
The ankle is made up of the tibia and fibula bones of the lower leg, and the tarsus bone of the foot held together by ligaments, which provide strength and stability during movement. The tibia and the fibula are held together by the syndesmotic ligaments, that allow appropriate physiologic motion between the tibia and fibula. The syndesmosis ankle injury (or high ankle sprain) is caused by external rotation of the talus and can occur in isolation or with a fibula fracture. Currently, syndesmotic fixation is performed with a screw or suture device. Each have their own risks and benefits. The screw provides rigid fixation however often must be removed or it will break due to micromotion. The suture device does not have to be removed but often does not provide enough rigid fixation or fixation in all planes to allow for appropriate healing.
Technical Summary
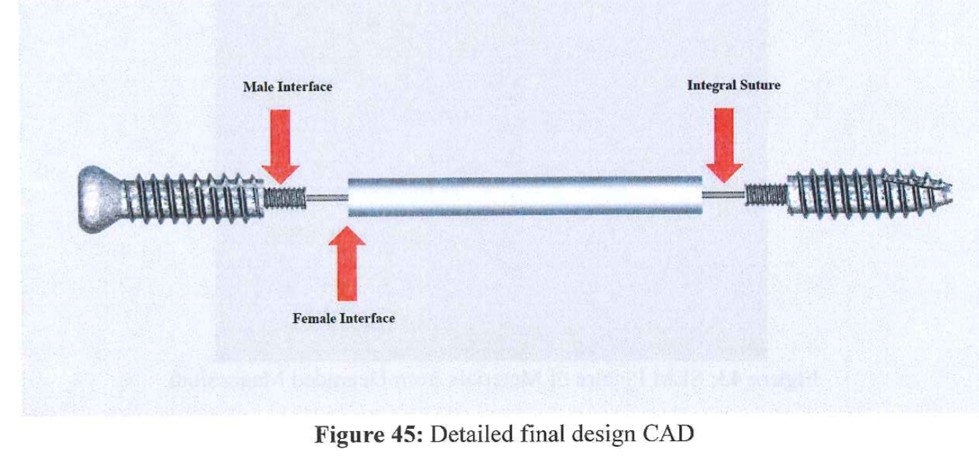
Emory researchers have developed a medical device which provides both rigid fixation initially while allowing for physiologic micromotion without hardware removal long-term. The invention makes use of a three-part screw which consists of a biodegradable core sandwiched between the head and the tip made of titanium alloy. The titanium tip and head are to provide X-ray detection, in case of complications and the need for removal. The biodegradable core was chosen due to its ability to degrade in 6-8 weeks, providing the flexibility needed after the syndesmosis ligament has healed without the need for a second surgery to remove the screw.
Developmental Stage
The project is currently at the design phase.
Patent Information
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Patent Status |
|
|

