Combination Drug Treatment for Neoplasms Associated with Tuberous Sclerosis
Application
Combination of rapamycin and imatinib for the treatment of neoplasms associated with tuberous sclerosis (TS).
Key Benefits
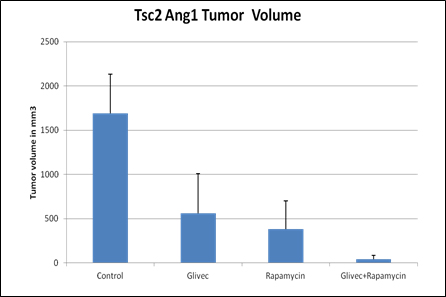
- Combined therapy shows 97% decrease in tumor volume in mouse model of TS compared to vehicle treatment.
- Dual therapy blocks two major signaling pathways implicated in TS.
Market Summary
Tuberous sclerosis (TS) is a rare genetic disease. The disease is characterized by benign neoplasm tumors in various organs including the brain, kidney, skin, heart, and lungs. Neoplasms can lead to the development of epilepsy, lesions of the kidneys and heart, and significant physical disfigurement. While symptoms can be mild, severe cases do exist and multiple renal lesions are the most common cause of morbidity in TS. Currently no effective medical treatment exists.
Technical Summary
Tuberous sclerosis is an autosomal dominant disorder related to two genes, hamartin (tsc1) and tuberin (tsc2). These genes are large, resulting in numerous potential treatment targets. Although inhibition of the mTOR pathway has been an initial treatment target for TS, clinical data suggest that multiple signaling pathways may be involved. One of these alternative targets is the platelet-derived growth factor β receptor (PDGFRβ), and studies have shown an inverse relationship between mTOR and PDGFRβ in TS cells. This inverse relationship suggests that mTOR and PDGFRβ may compensate for one another. Emory researchers have found that combined inhibition of mTOR and PDGFRβ with rapamycin and imatinib (Glivec) leads to a greater reduction in tumor volume than monotherapy of either rapamycin or imatinib, in a well-validated mouse model of TS.
Developmental Stage
Rapamycin is currently undergoing multiple phase 2 clinical trials for treatment of TS. Imatinib is a repurposed drug that has been approved by the FDA for use in treating other diseases.
Patent Information
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Patent Status |
| Utility (parent) |
United States |
13/357,920 |
8,691,777 |
1/25/2012 |
4/8/2014 |
Issued |
|
|

